Adrenogenital Syndrome
Content of This Page
1- Introduction
2- Causes
3- Symptoms
4- Investigations & Lab Results
5- Prognosis
6- Treatment
Introduction
Adrenogenital Syndrome, known as Congenital Adrenal Hyperplasia, refers to a group of inherited enzyme deficiencies that affect cortisol biosynthesis in the adrenal cortex. The most common form is 21-hydroxylase deficiency, accounting for over 90% of cases. This enzyme defect leads to low cortisol and often aldosterone, causing the pituitary to increase ACTH secretion, which stimulates the adrenal glands and results in excess androgen production. The clinical manifestations depend on the severity of enzyme deficiency and may include virilization of external genitalia in females, precocious puberty in males, salt-wasting crisis, and growth abnormalities.
Causes
The primary cause is genetic mutations leading to enzyme deficiencies involved in adrenal steroid synthesis. These include:
21-hydroxylase deficiency (most common – >90% of cases)
Caused by mutations in the CYP21A2 gene
Leads to ↓ cortisol and often ↓ aldosterone, with ↑ androgen production
11β-hydroxylase deficiency
Caused by mutations in the CYP11B1 gene
Leads to ↑ androgens and accumulation of 11-deoxycorticosterone (causing hypertension)
17α-hydroxylase deficiency
Caused by mutations in the CYP17A1 gene
Leads to ↓ sex steroids and cortisol, with ↑ mineralocorticoids (causing hypertension and hypokalemia)
3β-hydroxysteroid dehydrogenase deficiency
A rare form affecting the production of all steroid hormones (glucocorticoids, mineralocorticoids, and androgens)
Congenital adrenal hypoplasia (rare)
A structural defect of the adrenal glands, not enzymatic
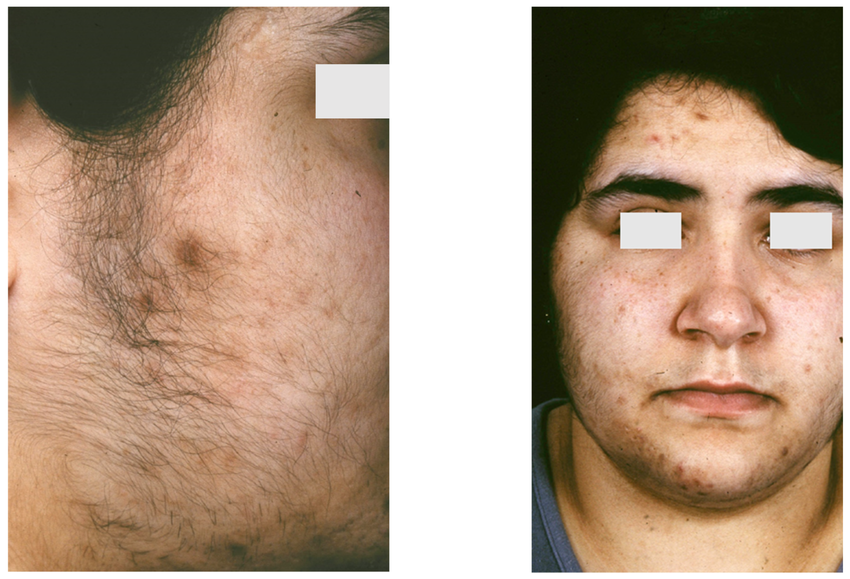
Symptoms
-In Females:
Ambiguous genitalia (virilization) at birth
Clitoromegaly, labial fusion
Normal internal female organs (ovaries, uterus)
Early appearance of pubic hair and acne
-In Males:
Normal genitalia at birth, may appear slightly enlarged
Signs of precocious puberty (early pubic hair, rapid growth, deep voice) in childhood
-In Both Genders:
Salt-wasting crisis (in salt-wasting form):
Vomiting, dehydration
Hypotension
Hyponatremia, hyperkalemia
Can be life-threatening if untreated
Poor weight gain or failure to thrive
Hypoglycemia (due to cortisol deficiency)
-Non-classic (Milder form):
May present later in childhood or adolescence
Early pubic hair, acne, and body odor
Accelerated growth and advanced bone age
Irregular menstruation or infertility in females
Hirsutism (excess facial/body hair)
Mild virilization without salt-wasting
Investigations & Lab Results
1. Serum Electrolytes:
↓ Sodium (Hyponatremia)
↑ Potassium (Hyperkalemia)
↓ Blood pressure (due to aldosterone deficiency in salt-wasting form)
2. Hormonal Assays:
↑ 17-hydroxyprogesterone (key screening and diagnostic marker)
↑ ACTH (due to cortisol deficiency → feedback stimulation)
↓ Cortisol
↓ Aldosterone (in salt-wasting form)
↑ Androgens (testosterone, DHEA) → leads to virilization
↑ Plasma renin activity (due to aldosterone deficiency)
3. ACTH Stimulation Test (Synacthen test):
Used to confirm diagnosis
Exaggerated ↑ in 17-hydroxyprogesterone after ACTH stimulation
4. Genetic Testing:
Mutations in the CYP21A2 gene (confirms 21-hydroxylase deficiency)
Useful for prenatal diagnosis and family screening
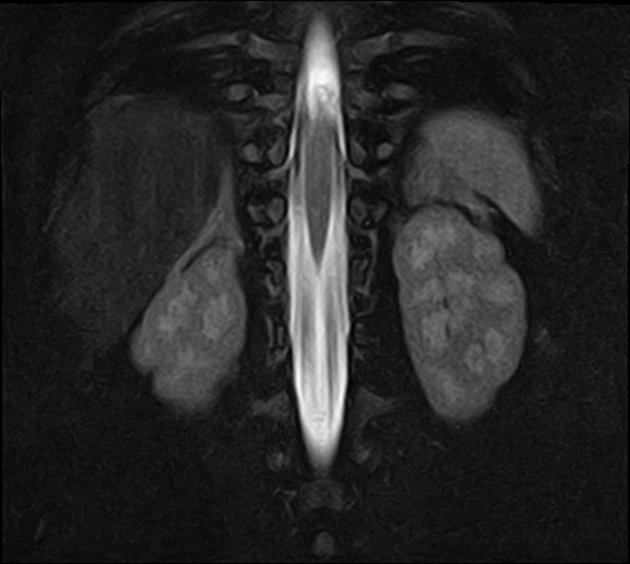
5. Imaging:
Pelvic ultrasound (in females):
Shows normal uterus and ovaries (to differentiate from other DSDs)
Prognosis
- Prognosis is excellent with early diagnosis and proper treatment using hormone replacement.
- Without treatment, infants may experience life-threatening dehydration, hypotension, and electrolyte imbalance.
- Simple virilizing form:
- Lifelong hormonal therapy ensures good health outcomes.
- May face issues like early puberty, short adult stature, or reduced fertility if not well-managed.
- Non-classic (late-onset) form:
- Usually mild with very good prognosis.
- May present later in life with symptoms like irregular periods, hirsutism, or acne, especially in females.
- Often does not require continuous treatment.
- Fertility:
- Can be reduced in some individuals, especially females, but is often improved with treatment.
- Psychosocial aspects:
- Early genital virilization and lifelong medical care may have psychological impact; multidisciplinary support is helpful.
Treatment
1. Glucocorticoid Replacement:
Hydrocortisone (preferred in children) or Prednisolone/Dexamethasone (in older patients)
Suppresses excess ACTH and reduces androgen overproduction
2. Mineralocorticoid Replacement (for salt-wasting forms):
Fludrocortisone
Helps maintain sodium balance, blood pressure, and fluid volume
3. Sodium Supplementation (Infants):
Especially important in the first year of life
Helps prevent dehydration and salt-wasting crisis
4. Surgical Management (if needed):
Genital reconstructive surgery in virilized females (controversial and individualized)
5. Monitoring and Follow-up:
Regular monitoring of:
Growth and development
Electrolytes, hormone levels (17-hydroxyprogesterone, renin, androgens)
Bone age (to detect early closure of growth plates)
6. Stress Dose Steroids:
During illness, surgery, or injury, increase steroid dose to prevent adrenal crisis
7. Genetic Counseling:
For affected families and carriers
Important for future family planning and prenatal diagnosis