Neuroblastoma
Content of This Page
1- Introduction
2- Causes
3- Symptoms
4- Investigations & Lab Results
5- Prognosis
6- Treatment
Introduction
Neuroblastoma is a malignant tumor of the sympathetic nervous system, arising from neural crest cells that form the adrenal medulla and sympathetic ganglia. It is the most common extracranial solid tumor in children, typically occurring in infants and young children under 5 years of age.
The tumor most commonly originates in the adrenal glands, but it can also arise along the sympathetic chain in the neck, chest, abdomen, or pelvis. Neuroblastoma is known for its variable clinical behavior, ranging from spontaneous regression to aggressive metastatic disease. It can secrete catecholamines, leading to specific biochemical markers (e.g., VMA, HVA in urine).
Causes
1. Genetic Mutations (Sporadic Cases – most common):
Somatic mutations in neural crest-derived cells
Often occur randomly and are not inherited
2. Familial Neuroblastoma (rare – ~1% of cases):
Autosomal dominant inheritance with incomplete penetrance
Associated with germline mutations in genes such as:
ALK (Anaplastic Lymphoma Kinase gene)
PHOX2B (paired-like homeobox gene)
3. Chromosomal Abnormalities:
MYCN gene amplification → associated with aggressive disease and poor prognosis
Loss of heterozygosity at 1p and 11q regions
Gain of 17q often found in high-risk tumors
4. Developmental Factors:
Errors during development of sympathetic nervous tissue in the embryo
Tumor originates from primitive sympathetic neuroblasts
5. Environmental Factors:
No consistent environmental or maternal exposure has been conclusively linked to neuroblastoma
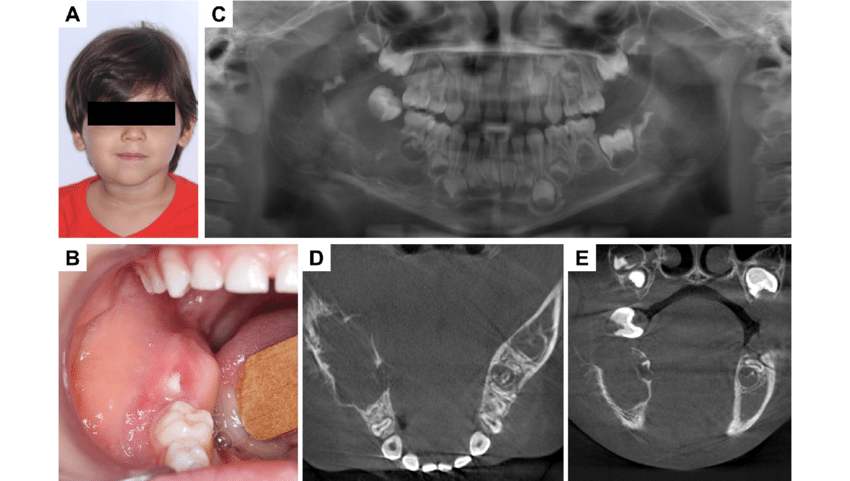
Symptoms
1. Abdominal Tumor (most common site: adrenal gland)
Abdominal mass or swelling (often palpable)
Abdominal pain or discomfort
Constipation or bowel obstruction (if tumor compresses intestines)
Distended abdomen
2. Systemic Symptoms
Fever
Weight loss and failure to thrive
Fatigue and irritability
3. Signs of Metastasis
Bone pain or limping (bone metastases)
Swelling or lumps in neck, chest, or elsewhere due to lymph node or soft tissue spread
Periorbital bruising or “raccoon eyes” (due to orbital metastases)
Proptosis (bulging of the eye)
Spinal cord compression symptoms (weakness, paralysis) if tumor involves spinal canal
4. Catecholamine-related Symptoms
Hypertension (rare)
Sweating, flushing, rapid heartbeat (due to catecholamine secretion)
5. Paraneoplastic Syndromes (less common)
Opsoclonus-myoclonus syndrome (dancing eyes/dancing feet) – a neurologic disorder associated with neuroblastoma
Investigations & Lab Results
1. Laboratory Tests:
Urine Catecholamine Metabolites:
↑ Vanillylmandelic acid (VMA)
↑ Homovanillic acid (HVA)
(Elevated in about 90% of neuroblastoma cases; useful for diagnosis and monitoring)
Complete Blood Count (CBC):
May show anemia or thrombocytopenia if bone marrow involvement
Lactate Dehydrogenase (LDH) and Ferritin:
Often elevated; markers of tumor burden
2. Imaging:
Ultrasound:
First-line imaging for abdominal masses; shows a heterogeneous mass
Computed Tomography (CT) Scan / Magnetic Resonance Imaging (MRI):
Defines tumor size, extent, and involvement of adjacent structures
Detects metastases in chest, abdomen, pelvis, and spine
Metaiodobenzylguanidine (MIBG) Scan:
Nuclear medicine scan specific for neuroblastoma cells
Detects primary tumor and metastatic sites with high sensitivity
Bone Scan:
Used to detect bone metastases if MIBG scan is not available or inconclusive
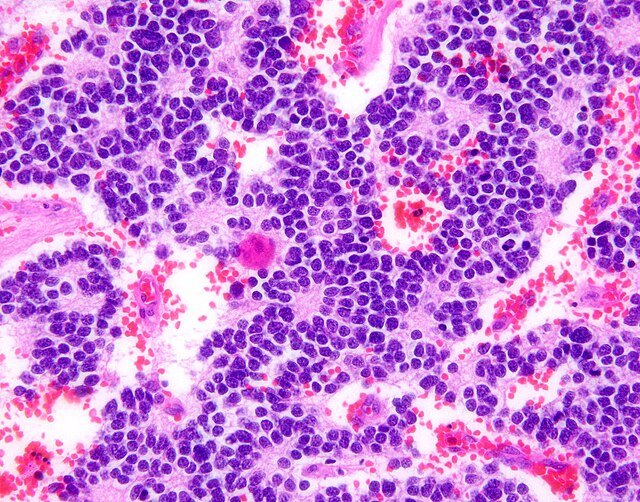
3. Biopsy and Histopathology:
Tissue biopsy confirms diagnosis
Shows small round blue cells with neuroblastic differentiation
Immunohistochemistry positive for markers like neuron-specific enolase (NSE), synaptophysin, and chromogranin
4. Bone Marrow Aspiration and Biopsy:
To detect marrow infiltration by tumor cells
5. Genetic and Molecular Testing:
MYCN amplification (poor prognosis marker)
Chromosomal abnormalities (1p deletion, 11q deletion, 17q gain)
Prognosis
-Highly variable depending on several factors:
Age at diagnosis
Stage of disease
Tumor biology (e.g., MYCN gene status)
Histopathological features
-Favorable Prognostic Factors:
Age younger than 18 months at diagnosis
Localized disease (Stages 1 and 2)
Tumors without MYCN amplification
Differentiated tumor histology
Low-risk genetic features
-Unfavorable Prognostic Factors:
Age over 18 months
Metastatic disease (Stages 3 and 4)
MYCN gene amplification (associated with aggressive tumors)
Unfavorable histology (undifferentiated or poorly differentiated)
Chromosomal abnormalities such as 1p and 11q deletions
-Outcome:
Excellent survival rates (>90%) with appropriate treatmen
Treatment
Surgery:
Main treatment for localized tumors to remove the primary mass.
May also be done for biopsy or to reduce tumor burden in advanced cases.
Chemotherapy:
Used for intermediate and high-risk neuroblastoma to shrink tumors and treat metastases.
Common agents include cyclophosphamide, vincristine, doxorubicin, cisplatin, and etoposide.
Radiation Therapy:
Applied for residual disease after surgery or metastatic sites, especially in older children and high-risk cases.
High-Dose Chemotherapy with Stem Cell Transplant:
Used in high-risk patients after initial chemotherapy to improve outcomes.
Immunotherapy:
Anti-GD2 monoclonal antibodies (e.g., dinutuximab) combined with cytokines and isotretinoin to reduce relapse risk in high-risk cases.
Supportive Care:
Management of symptoms such as pain, spinal cord compression, or hormonal effects.
Observation:
Some low-risk or infant cases may be closely observed due to potential spontaneous regression.