G6PD Deficiency
Content of This Page
1- Introduction
2- Pathophysiology
3- Genetics & Inheritance
4- Triggers & Precipitating Factors
5- Clinical Features
6- Investigations
7- Treatment
8- Prognosis & Follow-up
9- What Should You Avoid
Introduction
Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency is the most common enzyme deficiency worldwide, affecting over 400 million people. It is an X-linked recessive disorder that increases red blood cell (RBC) vulnerability to oxidative stress, leading to episodic haemolysis when triggered.
This condition is particularly important in clinical practice due to its:
High prevalence in malaria-endemic regions (e.g. Africa, Asia, Mediterranean),
Triggering by common drugs or foods, and
Role in neonatal jaundice and acute haemolytic anaemia.
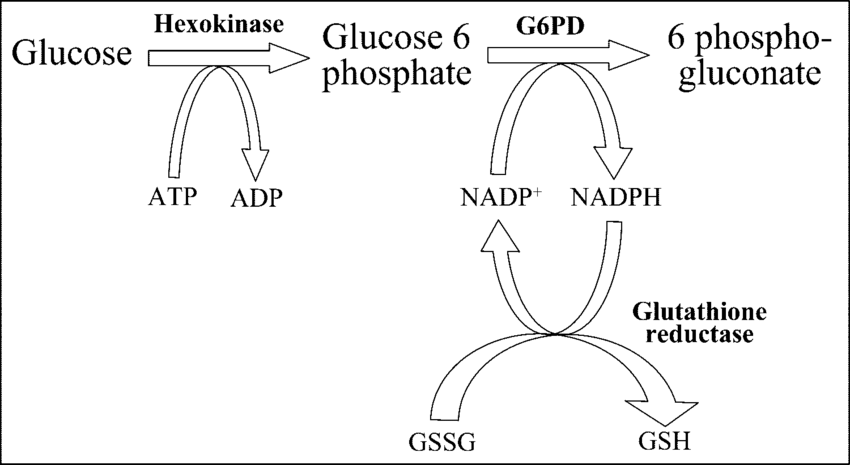
Pathophysiology
1. Role of G6PD in Red Cells
G6PD is part of the pentose phosphate pathway (also called the hexose monophosphate shunt).
It generates NADPH, which is required to:
Keep glutathione in its reduced (active) form.
Detoxify hydrogen peroxide (H₂O₂) and other reactive oxygen species (ROS).
2. What Happens in G6PD Deficiency?
Without sufficient G6PD:
NADPH production falls.
Reduced glutathione is depleted.
RBCs cannot neutralize oxidative agents.
3. Oxidative Damage to RBCs
Exposure to oxidative stress (e.g. infections, drugs, fava beans) leads to:
Denaturation of haemoglobin, forming Heinz bodies.
Damage to the red cell membrane → RBCs become rigid and are removed in the spleen (extravascular haemolysis) or lyse in the circulation (intravascular haemolysis).
4. Haemolysis and Clinical Symptoms
Sudden drop in haemoglobin (acute haemolytic anaemia).
Jaundice due to excess bilirubin from RBC breakdown.
Dark urine (haemoglobinuria) in severe cases.
Genetics & Inheritance
1. Inheritance Pattern: X-Linked Recessive
Males (XY): Only one X chromosome → affected if they inherit the defective gene.
Females (XX): Two X chromosomes → usually asymptomatic carriers, unless:
Homozygous (rare),
Or show skewed X-inactivation (lyonisation), making them functionally deficient.
Thus, males are more commonly and severely affected, while females are usually carriers.
2. Transmission Risks
| Parental Genotype | Offspring Risk |
|---|---|
| Carrier mother × normal father | 50% of sons affected, 50% of daughters carriers |
| Affected father × normal mother | No affected sons, all daughters carriers |
| Affected father × carrier mother | Can produce affected sons and homozygous daughters |
3. Variants and Geographic Distribution
There are over 400 known G6PD variants with varying enzyme activity levels.
Common variants:
G6PD A- (mild/moderate deficiency) – common in African populations.
G6PD Mediterranean (severe deficiency) – common in Middle East, South Asia, and southern Europe.
Prevalence is high in malaria-endemic regions due to a protective effect against Plasmodium falciparum.
4. Clinical Implications of Genetics
Severity of deficiency (and thus risk of haemolysis) varies by variant.
Genetic testing may be used in unclear cases or in female carriers.
Triggers & Precipitating Factors
| Trigger Type | Examples |
|---|---|
| Drugs | Sulfonamides, dapsone, primaquine, nitrofurantoin |
| Infections | Any febrile illness (especially bacterial or viral) |
| Foods | Fava beans (favism) |
| Chemicals | Naphthalene (mothballs), industrial oxidants |
| Other | Stress (e.g. surgery, childbirth) |
Clinical Features
| Clinical Scenario | Key Features |
|---|---|
| Neonate | Prolonged jaundice, risk of kernicterus |
| Acute haemolysis (triggered) | Sudden pallor, jaundice, dark urine, fatigue |
| Chronic haemolysis (rare) | Mild persistent anaemia, jaundice |
| Asymptomatic carrier | No signs unless stressed by oxidants |
Investigations
1. Initial Tests: Confirming Haemolysis
| Test | Expected Findings |
|---|---|
| Full blood count (FBC) | Normocytic anaemia ± reticulocytosis |
| Reticulocyte count | ↑ (compensatory marrow response) |
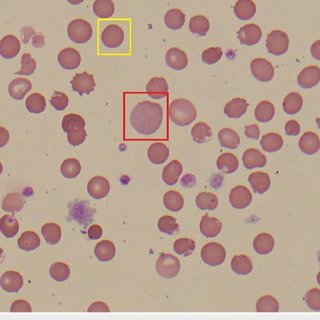
| Peripheral blood film | “Bite cells” and blister cells (from splenic pitting of Heinz bodies) |
| Unconjugated bilirubin | ↑ due to red cell breakdown |
| Lactate dehydrogenase (LDH) | ↑ marker of cell turnover |
| Haptoglobin | ↓ (consumed during intravascular haemolysis) |
| Urinalysis | May show haemoglobinuria, especially in severe intravascular haemolysis |
2. Specific Test: G6PD Enzyme Assay
Confirms diagnosis by measuring G6PD activity in red cells.
Important note:
Avoid testing during acute haemolysis, as older, G6PD-deficient cells are destroyed first.
Recent transfusion may also mask deficiency (donor RBCs can carry normal G6PD).
Best done 2–3 weeks after recovery, when the red cell population stabilizes.
3. Additional/Confirmatory Testing (if needed)
| Test | Use |
|---|---|
| Heinz body stain | Detects denatured haemoglobin (requires special staining) |
| Molecular genetic testing | Identifies G6PD mutations (e.g. in neonates or family screening) |
Treatment
| Scenario | Management |
|---|---|
| No symptoms | Avoid triggers, educate patient |
| Acute haemolysis | Supportive care, hydration, transfusion if needed |
| Neonatal jaundice | Phototherapy ± exchange transfusion |
| Infection-triggered haemolysis | Prompt antibiotics, monitor renal function |
Prognosis & Follow-up
1. Prognosis
| Clinical Form | Prognosis |
|---|---|
| Asymptomatic carrier (most common) | Excellent—normal lifespan, no complications |
| Intermittent acute haemolysis | Very good if triggers are avoided |
| Favism (fava bean sensitivity) | Can be life-threatening but preventable |
| Chronic non-spherocytic haemolytic anaemia (rare) | Variable; may require ongoing monitoring |
| Neonatal jaundice (G6PD-related) | Good if recognised and treated early (e.g. phototherapy) |
2. Follow-Up Recommendations
a. Initial Diagnosis Phase
Confirm enzyme deficiency (after acute crisis has resolved).
Identify and document safe vs unsafe drugs.
Educate about food, chemical, and infection-related triggers.
b. Routine Follow-Up (As Needed)
| Component | When it’s required |
|---|---|
| Retesting G6PD activity | If initial test during crisis gave normal result |
| Haemoglobin checks | If patient has chronic or recurrent haemolysis |
| Renal function monitoring | After severe haemolysis episodes |
| Genetic counselling | In affected families or for prospective parents |
3. Patient Education & Prevention
Provide a written list of:
Unsafe medications (e.g. sulfonamides, dapsone, primaquine)
Unsafe foods (e.g. fava beans)
Encourage early medical attention if symptoms of haemolysis appear:
Sudden fatigue
Jaundice
Dark urine
What Should You Avoid
| Avoid | Examples |
|---|---|
| Drugs | Primaquine, dapsone, sulfonamides, nitrofurantoin |
| Foods | Fava beans |
| Chemicals | Naphthalene (mothballs) |
| Stressors | Infections, fever, fasting |