Surface tension of water by the capillary tube method
Content of this page :
1. Introduction of the experiment.
2. Aim of the experiment.
3. Tools of the experiment.
4. Steps and methods of the experiment.
5. Parameter, Theory and Final law of the experiment.
6. Table of The Readings.
7. Medical application and advantages of the experiment.
1. Introduction of the experiment:
Surface Tension is a property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This phenomenon can be observed in the nearly spherical shape of small drops of liquids and of soap bubbles. Because of this property, certain insects can stand on the surface of water. The high surface tension of water is caused by strong molecular interactions. The surface tension arises due to cohesive interactions between the molecules in the liquid. At the bulk of the liquid, the molecules have neighboring molecules on each side.
2. Aim of the experiment:
-To measure the surface tension of water by capillary tube method.

3-Tools of The experiment
· Set of three glass capillary tubes-one of mm diameter. One of greater and one of less diameter.
· Dilute nitric acid.
· Dilute caustic soda solution.
· Travelling microscope or glass scale.
· Rubber bands.
· Beaker.
· Stand and clamp.
· Thermometer.
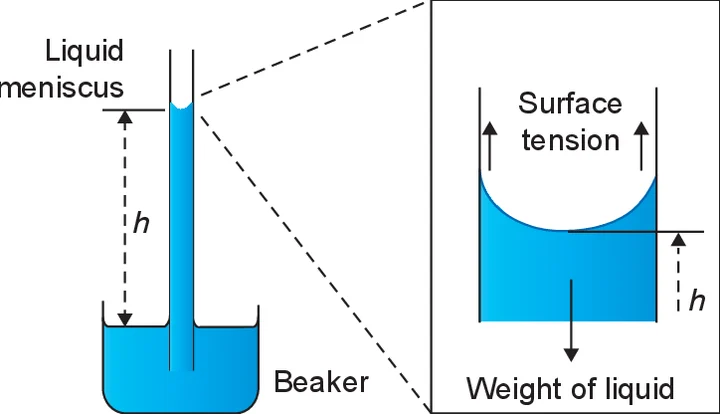
4. Steps and methods of the experiment:
lean all three capillary tubes free from dirt and grease both inside and outside by washing them successively with nitric acid, with tap-water, with caustic soda solution and lastly and repeatedly with tap- water. The beaker and the glass scale (if used) must also be free from dirt and grease so clean them in the same way.
Select the capillary tube of medium bore and attach a bent pin to it by means of a rubber band. Hold the capillary tube in a clamp with its lower end immersed in the water. Before measuring the capillary rise push the tube a little farther down into the water and then restore it to its original position. This ensures that the tube is wet a little above the meniscus.
Adjust the position of the bent pin until its point just touches the water surface. Focus the microscope on the meniscus of the water level in the capillary tube and adjust the microscope until the horizontal cross- wire is tangential to the bottom of the meniscus which is of course seen inverted in the eyepiece of the microscope. To facilitate preliminary focusing of the microscope on the meniscus it is useful to hold a piece of paper with printing on it behind the capillary tube and first focus on that. Record the position of the travelling microscope on its vertical scale (h1).
Mark the position of the meniscus on the capillary tube with a loop of cotton or by gummed paper and then carefully remove the beaker of water from contact with the bent pin. Lower the microscope until it can now be focused on the tip of the bent pin. Record the position of the travelling microscope on its vertical scale (h2).
With the aid of a file cut the capillary tube at the place previously occupied by the meniscus and measure the internal diameter d by the travelling microscope, taking the mean of two determinations at right angles. Repeat all the measurements with the other two clean capillary tubes in turn.
Record the temperate of the water.
5-Parameters, Final Law of The experiment
Parameters:
𝑦: Surface tension in (𝑁𝑚−1)
𝑑: Diameter of the capillary tube in (𝑚)
ℎ: Height of the water inside capillary tube in (𝑚)
𝜌: Density of water (1000kg𝑚−3)
𝑔: Gravitational acceleration (9.8 𝑚𝑠 −3)
Final Law:
Surface Tension 𝒚 = 𝟏/𝟒 𝒅𝒉𝝆𝒈
6. Table of the Readings:

7 -Medical Application:
In medicine, surface tension measurement is above all used in connection with various pathological states of lung surfactants such as adult respiratory distress syndrome, bronchial asthma, and pneumonia.