Charcot-Marie-Tooth disease
Content of This Page
1- Introduction
2- Causes
3- Symptoms
4- Investigations & Lab Results
5- Complications
6- Treatment
Introduction
Charcot-Marie-Tooth disease is a group of inherited disorders that affect the peripheral nervous system, which includes the nerves outside the brain and spinal cord. It causes progressive weakening and wasting of the muscles, especially in the feet, lower legs, hands, and forearms, along with loss of sensation in these areas.
The disease typically begins in childhood or early adulthood, although onset can vary. It is slowly progressive and results from mutations in genes that affect the structure and function of peripheral nerves. Despite its name, Charcot-Marie-Tooth disease is not related to the teeth.
Although it is a chronic condition, it does not usually affect life expectancy, and many people lead active lives with appropriate management and support.
Causes
1. Genetic Inheritance Patterns
CMT is inherited in several ways:
Autosomal dominant (most common)
Autosomal recessive
X-linked
Each subtype is associated with specific gene mutations and inheritance patterns.
2. Main Types and Associated Genes
| CMT Type | Primary Defect | Common Mutated Genes |
|---|---|---|
| CMT1 | Demyelinating neuropathy | PMP22 (duplication), MPZ, LITAF |
| CMT2 | Axonal neuropathy | MFN2, GARS, GDAP1 |
| CMTX | X-linked (usually in males) | GJB1 (encoding connexin 32) |
| CMT4 | Autosomal recessive | SH3TC2, GDAP1, MTMR2, others |
3. Pathophysiology
CMT1: Abnormalities in myelin sheath cause slow nerve conduction
CMT2: Damage to the axon causes reduced nerve signal strength
CMTX: Combination of both axonal and demyelinating features
4. Sporadic Cases
Rare, but some individuals develop CMT without a family history due to de novo mutations

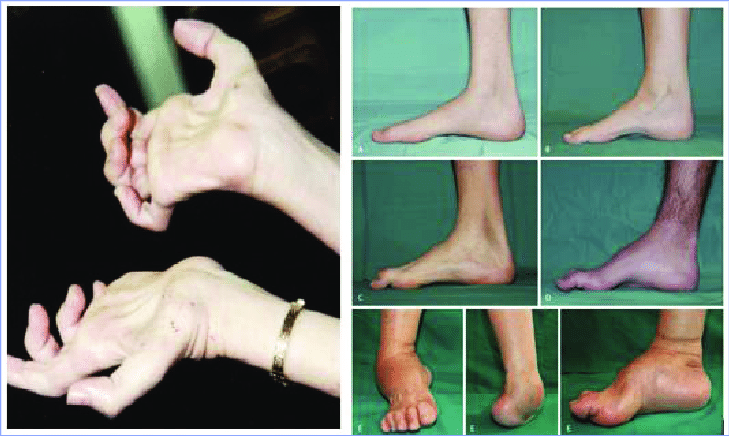
Symptoms
1. Muscle Weakness and Atrophy
Foot and lower leg muscles are most commonly affected
Difficulty lifting the front part of the foot (foot drop)
Frequent tripping, clumsiness, or abnormal walking patterns
Calf muscle wasting, leading to “inverted champagne bottle” appearance
Later involvement of hand and forearm muscles (grip weakness, fine motor difficulties)
2. Sensory Loss
Decreased sensation in the feet, legs, hands, or arms
Numbness, tingling, or burning sensations
Poor proprioception (reduced sense of position and movement)
3. Foot Deformities
High arches (pes cavus)
Hammertoes
Flat feet or other skeletal deformities due to muscle imbalance
4. Gait Abnormalities
Difficulty running or climbing stairs
Steppage gait due to foot drop
5. Reflex Changes
Decreased or absent deep tendon reflexes, especially at the ankles
6. Progression
Slowly progressive
May lead to significant disability over time, though many patients remain ambulatory
Investigations & Lab Results
1. Clinical Evaluation
Family history of neuropathy
Physical exam shows distal muscle weakness, atrophy, foot deformities (e.g., pes cavus), and reduced reflexes
2. Nerve Conduction Studies (NCS) and Electromyography (EMG)
These are essential to confirm peripheral neuropathy and to classify the type:
CMT1 (Demyelinating)
Markedly slowed nerve conduction velocities (NCVs) (<38 m/s in motor nerves)
Prolonged distal latencies and conduction blocks
CMT2 (Axonal)
Normal or mildly reduced NCVs
Low amplitude responses due to axonal loss
CMTX
Mixed features, often intermediate conduction velocity
3. Genetic Testing
Confirms the diagnosis and identifies the specific mutation
Often targets common genes first (e.g., PMP22 duplication for CMT1A)
Helpful for family counseling and prenatal diagnosis
4. Laboratory Tests
Usually normal, but used to exclude acquired causes of neuropathy:
Fasting blood glucose/HbA1c (for diabetic neuropathy)
Vitamin B12 levels
Thyroid function tests
Serum protein electrophoresis (rule out paraproteinemic neuropathy)
5. Nerve Biopsy (rarely needed)
May be used in unclear cases
Shows onion bulb formation in demyelinating types (CMT1) due to repeated demyelination and remyelination
6. Imaging (optional)
MRI of lower legs may show fatty replacement of muscles
Used to assess the extent of muscle atrophy
Complications
1. Mobility Impairment
Progressive muscle weakness, especially in the feet and lower legs, can lead to:
Difficulty walking
Frequent tripping or falls
Need for assistive devices (e.g., braces, walkers, wheelchairs)
2. Foot and Hand Deformities
Common due to muscle imbalance:
Pes cavus (high-arched feet)
Hammertoes
Flat feet
Claw hand deformity in later stages
3. Loss of Fine Motor Skills
Weakness in the hands can make tasks like writing, buttoning clothes, or using tools difficult
4. Chronic Pain and Cramps
Neuropathic pain due to nerve damage
Muscle cramps and fatigue are common
5. Sensory Impairment
Loss of sensation increases risk of:
Unnoticed injuries (e.g., burns, cuts)
Balance issues due to loss of proprioception
6. Psychosocial Effects
Anxiety, depression, and reduced self-esteem due to physical limitations and visible deformities
Social isolation in severe or visibly disabling cases
7. Complications from Surgery or Bracing
Orthopedic surgery (e.g., foot correction) carries risks of infection, delayed healing
Braces may cause skin irritation or pressure sores
Treatment
1. Physical and Occupational Therapy
Physical therapy:
Strengthening and stretching exercises to maintain muscle tone and flexibility
Prevent contractures and deformities
Improve balance and gait
Occupational therapy:
Helps with fine motor tasks and daily living activities
Adaptive tools for dressing, writing, eating, etc.
2. Orthopedic Interventions
Braces and orthotics:
Ankle-foot orthoses (AFOs) to correct foot drop and improve walking stability
Custom shoes or insoles for foot deformities
Surgical treatment (if needed):
Correct severe foot deformities (e.g., pes cavus, hammertoes)
Tendon transfers or osteotomies for better alignment
3. Pain Management
Neuropathic pain medications:
Gabapentin, pregabalin, amitriptyline, or duloxetine
Over-the-counter analgesics for muscle aches and cramps
4. Genetic Counseling
Recommended for affected individuals and families
Helps understand inheritance patterns and assess risk in offspring
5. Regular Monitoring and Support
Routine follow-up to monitor progression
Psychosocial support for emotional well-being
School and workplace accommodations if needed
6. Experimental and Emerging Therapies
Ongoing research into gene therapy and molecular treatments
Not yet available as standard care